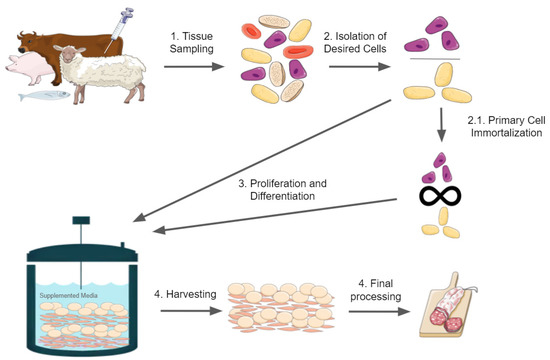
3.2. The Food Safety of Cell Lines
One of the most common concerns about cultured meat from consumers is the safety of ingesting cell lines. Currently, cultured animal cell lines are not commonly eaten by consumers. An immortal cultured meat cell line may contain expressed oncogenes, and if so, food products produced with these cells will need to be confirmed not to have tumorigenicity [43,44]. Although there is limited evidence to suggest that DNA from genetically engineered plant cells can integrate or be transferred into somatic cells or the microflora of the human gastrointestinal tract [45], due diligence would require further investigation in genetically engineered animal cells. Confirming that future products made from immortalized animal cells expressing oncogenes, either through spontaneous immortalization or genetic engineering, would be safe represents a gap in knowledge in this field. In addition, future immortalized cells should also undergo physico-chemical inspection throughout the production process, and ultimately be tested for safe consumption. Cells expressing novel levels of non-native compounds, such as enhancement with carotenoids, will require special confirmation that these levels are safe for human consumption. Oncogene expression could be tested during the manufacturing process of cultured meat by sampling a patch from a small portion of the cells which should be an accurate representation of the entire population in a cell line [44,46]. In the case that a cell line is confirmed safe for consumption, it will still need to be regularly monitored for contamination and genetic drift. Contamination of cell lines with other cell lines and adventitious agents can be common in cell culture [44,46,47]. Animal-sourced components of cell culture media, such as fetal bovine serum, are frequently at risk of harboring adventitious agents [44]. In part because of these challenges (along with other issues such as high cost, limited availability, and animal welfare concerns), non-animal-origin reagents are being extensively studied for cultured meat [44]. Genetic drift is also a known occurrence in cell lines where, over time, mutations build up that eventually cause changes in phenotypes [48]. To mitigate the risk of losing cell line fidelity to genetic drift, cell banks must be built in which cryopreserved cultures of relevant cell lines are quality-controlled and protected against the presence of viruses, bacteria, yeast, and mycoplasma. Such banks may include both a master cell bank, a collection of cells of uniform composition derived from a single source, and a working cell bank, a collection of cells derived from one or more vials of cells from the master bank expanded by serial subculture for use in cultivation. To create a cell bank, cells will need to be selected, validated, and cryopreserved in small batches that can later be thawed, validated again, and expanded for cultivation [44]. Cryopreservation techniques should be used that are animal-free and confirmed safe for cultured meat. Banked cells will be stored in multiple locations at ultra-low temperatures, likely under liquid nitrogen. During storage, there may be the potential for contamination by liquid nitrogen transferring pathogens to cells or cross contamination due to the leakage of cryopreservation bags. Storage in the vapor phase rather than the liquid phase might reduce the potential for otherwise possible cross-contamination, because liquid nitrogen has the potential to transfer pathogens to cells, even if stored in freezing bags [44,49]. Cell line authentication and screening will be critical in controlling contamination [44]. Cultures can be compared to these preserved cell lines to ensure quality control and replaced with serial subcultures of the preservation vials [44,48]. Similar cell banks exist for vaccination cultures [50]. Regulatory agencies are beginning to adapt pathways for cultured meat products to be examined regarding safety for consumption. To some degree, this will involve updating the existing regulations on cell-derived products for relevance to consumption as food [51,52,53]. Although animal-cell-cultured food has not been marketed before, cell lines have been used previously to create food ingredients or additives, such as enzymes, oils, and transgenic proteins, and also for numerous cell therapies [51]. For cultured meat, the Singapore Food Agency (SFA) became the first national agency to approve a cultured meat product, a cultured chicken nugget made with fetal bovine serum, in December 2020 [54]. To receive approval, the chicken was reviewed by the SFA via a “novel food” petition, which included a description of the cultivation process, the nutritional composition and characterization of the final product, information related to cell lines, scaffolding, media, and safety assessments covering possible hazards as well as other relevant safety studies such as digestibility assays and allergenicity profiling [55]. The petition’s specific information on cell lines entailed a description of the cell line source, description of the modifications and how these relate to the expression of substances that may result in food safety risk, description of the methods used for selection, screening, preparation, and banking, and information on how the purity and genetic stability of cell culture is ensured during the manufacturing process [56]. In the United States, the Food and Drug Administration (FDA) has announced that they will conduct pre-market consultations with companies on a product basis to evaluate the safety of cell lines, as well as production materials/process, tissue collection, components, and inputs [57]. The consultations involving cell lines will include collaborations with the Food Safety and Inspection Service (FSIS, an agency of the United States Department of Agriculture), which will regulate the product after cell harvest. After products are marketed, the FDA will oversee initial cell collection, the development and maintenance of qualified cell banks, proliferation, and the differentiation of cells through time of harvest [56]. In the European Union, cell-cultured meat may be regulated under the Novel Foods Regulation, although cultured meat from engineered immortalized cell lines may be regulated under separate approval under Regulation No. 1829/2003 regulating foods containing or produced from GM organisms [57,58]. Regulatory bodies in a few other regions have begun to adapt their regulations to new cultured meat products [57].
3.3. Cell Lines and the Consumer Acceptance of Cultured Meat
The immortalization method itself may be an important factor in the decision of consumers to accept cultured meat. To the best of the authors’ knowledge, no study has yet compared the consumer acceptance of cultured meat made of primary cells and cultured meat made of immortalized cells. There have, however, been initial studies analyzing the acceptance of genetically modified and non-genetically modified cultured meat. These studies found that consumers are more willing to purchase non-genetically modified cultured meat compared to genetically modified cultured meat [59,60]. Products with genetic modifications for immortalization may therefore have lower consumer acceptance compared to similar products without genetic modifications, such as products made with primary cells, spontaneously immortalized cells, or cells immortalized by a footprint-free genetic engineering method with any gene manipulations removed from the final product. Consumer acceptance of food biotechnology has been found to be negatively affected by perceived unnaturalness (“tampering with nature” due to biological transformations), neophobia (fear of novel foods), and social distrust of the food industry [61]. For cultured meat in particular, distrust and the perceptions that it is unnatural or not real meat have been shown to be strong drivers in consumers’ willingness to eat cultured meat compared to the perceived benefits [11,61,62,63]. There are, however, some differences across applications and among consumers in how genetic engineering is evaluated that may inform decisions on the immortalization of cell lines for cultured meat. Consumers appear to be more concerned when genes are exchanged between different species, and see gene insertion as more unnatural than gene deletion [64,65,66]. Unless genetic engineering has tangible benefits for consumers (for example, cheaper, better tasting, nutritionally enhanced food), consumers’ negative perception of genetic modifications may have a compounding effect in lowering the acceptance of genetically modified cultured meat [67,68]. As mentioned in Section 1, the cell types and species that consumers may accept most readily for consumption differ from the type and species used in other cell line applications. Other cell line applications for myoblasts, for instance, use the myoblasts of model organisms such as mice, rat, hamsters, and Japanese quail [9], whereas these species are not typically eaten by consumers in many food cultures. Rather, the most commonly consumed animals globally are poultry, pigs, and cattle [68]. Food neophobia appears to be a major factor in many consumers’ acceptance of cultured meat, suggesting that cultured meat could find less acceptance if made from species and cell types that are unfamiliar to consumers [11,62,69,70,71,72,73]. Consumers’ preferred cell type and the extent of food neophobia will also differ across food cultures. Chinese and American consumers have been found to have lower food neophobia compared to Indian consumers and are accustomed to a more diverse array of meat [69]. As an example of preferred cell type, fish maw is relatively expensive and desirable in Chinese markets, but unfamiliar to Western consumers, making it a potentially more desirable target for cultured meat in China than in the West [73]. Furthermore, some cell lines may be prohibited from consumption under religious food restrictions. Jewish kosher laws and Islamic halal laws do not allow the consumption of conventional pork and other meats [74], and it is still be debated as to whether these laws may be similarly interpreted to not allow the consumption of cultured meat derived from the same species. These religious restrictions depend not only on species, but on the method of meat production [75]. Many Hindus do not eat conventional meat because they consider it against their principle of nonviolence, so they may not accept cultured meat produced with harm to animals [76]. Some rabbis and Islamic jurists have expressed that their rulings on cultured meat will depend on whether the cells are from a kosher-slaughtered animal or a halal-slaughtered animal, respectively, and whether production includes use of blood or serum [75,76,77,78,79].
3.4. Other Unique Attributes to Select for in Cultured Meat Cell Lines
Cell lines for cultured meat production will not only be from animals and cell types not previously established, but will also require different attributes from those previously typical for immortal cell lines. Specifically, they should be food-safe, able to proliferate stably and efficiently in a large-scale production environment with minimum costs, and have desirable taste, texture, and nutrition.
Multiple companies have filed patents on ways to overcome the unique challenges of growing food-relevant cells at scale. The cultured meat company Wild Type has filed one patent on a footprint-free method of genetic modifications to control cell differentiation and proliferation with a small genetic footprint [80]. Another company, Upside Foods, has filed one patent to genetically engineer their pig cell line O2K so that they can replace some growth factors with small molecules, reducing the need for expensive animal serum or supplemental biologics in the high volume of media required for cell production [81,82]. The same company has also filed a patent to genetically modify cell lines with an overexpression of glutamine synthetase to convert ammonia into an amino acid [83]. Ammonia build-up is a challenge for large-scale cell culture, because it has known inhibitory and toxic properties to cell culture and bioreactors cannot replicate the uptake of ammonia by the bloodstream as in a complete organism [84]. Overexpression of glutamine synthetase could also provide an additional amino acid source for cells [83]. Modifications such as these aim to make cultured meat cell lines safer, less expensive, and more efficient to culture at large scales [58,59,60].The sensory experience of cultured meat may be affected by the cell types used. A scientific paper on the sensorial qualities of cultured meat compared to animal-sourced meat has not been published thus far [85]. Although other inputs such as feed or media may likely affect the sensorial qualities of cultured meat, future research may indicate innate differences in taste and texture between cultured cell types [85,86]. The inclusion of cell types commonly found in conventional meat, including myofibers, fibroblasts, adipocytes, endothelial cells, and ECM-producing supporting cells, could improve the ability of cultured meat to produce similar tastes and textures to conventional meat [8,85]. In order to produce cultured meat with the mentioned cell types, cultured meat cell lines would either need to be made from these cell types or from satellite cells and adipose-derived stem cells, mesenchymal stem cells, or pluripotent stem cells able to be differentiated in vitro into these cell types [8,87]The sensory experience of cultured meat may additionally be affected by certain attributes to be selected for in cell lines. Consumer surveys have found that many consumers expect cultured meat to be less tasty than conventional meat, and this perception contributes to their unwillingness to consume cultured meat [11,12]. The sensory experience of meat generally involves taste and aroma from the Maillard reaction and lipid oxidation reactions, color from heme proteins, and a tender, juicy texture [85]. Techniques have begun to be studied in modifying the nutritional profile of cultured meat cell lines, and could also be applied to alter the taste and aroma of cultured meat by variation in the production of different flavor compounds within cells [88]. Cultured meat’s color is generally paler than conventional meat because the expression of the heme protein myoglobin is suppressed at ambient oxygen conditions. In order to recreate the color of conventional meat, myoglobin or another colorant could be directly added to cultured cells, cultivation could be adapted to low oxygen conditions, or cell lines could be engineered to express myoglobin at the oxygen level of cultivation [85,89]. Cultured meat’s texture would differ from that of structured meat unless adipose and muscle cells were able to be co-cultured together, either on a scaffold mimicking connective tissue or on an extracellular matrix made by the cells themselves [85]. In addition to the intrinsic taste of each cell, the ability of adipose and muscle cell lines to be co-cultured into thick tissue may become crucial for structured cultured meat products. Fat contributes to meat’s taste, aroma, juiciness, and tenderness, and successful co-culturing could create structured meats such as steaks or pork chops [90]. Thick tissue with strong binding is used for a number of meat products, and would require the use of external binders if cells are not able to create these structures themselves in vitro [85]. All these cell attributes together could affect the sensory experience of cultured meat [85,90]. Although no data are publicly available, several groups have announced plans to work with cells from specific heritage breeds, with the idea that genetics partially determines the sensory properties of meat [91,92,93]: Cell Farm Food Tech aimed to produce mesenchymal stem cell lines from their native Argentinian cattle breeds [94], and three groups are researching the use of cells from Wagyu cows to make cultured beef [87,95,96]. Further evaluations of how a donor animal’s species, age, gender, and provenance affect the taste and texture of cultured meat produced with its cells could help ensure that cultured meat is not only safe, but desirable to consumers [85,97]. Cultured meat should be designed to provide similar, if not enhanced, available levels of nutrients compared to conventional meat. Meat provides 26% of the global protein supply, 24% of the global fat supply, and 9% of the global calorie supply per capita per day, as well as essential micronutrients such as iron, zinc, and vitamins A and B12 [98]. For cultured meat to replicate the dietary place of meat, it must provide similar levels of these nutrients through some combination of components including cells, scaffolding, and added nutrients. The combination of these components will contribute to the nutritional composition as well as the bioavailability of these nutrients, because the ability to digest, absorb, and metabolize nutrients is affected by the matrix in which they are incorporated. Cell line attributes relevant for nutrition can therefore be categorized as the following: their ability to co-culture with other cell types to achieve a nutritional composition similar to conventional meat, their ability to take up nutrients added to its media to supplement the nutrition of the end product, their specific percentage content of fat and protein, their fatty acid and amino acid composition, and the bioavailability of their nutrients depending on the matrix within which it is consumed [75,85]. Some important nutrients in conventional meat, including essential fatty acids and vitamin B12 and minerals, are not produced in muscle cells but are derived from animal feed components which have been digested and modified by non-muscle organs. Unless specifically added to the culture medium and taken up by the cells, or added in post-harvest processing (Figure 1), these compounds would be absent from cultured meat, influencing nutrition [85].Other cultured meats could be engineered for desired differences from the nutritional profile of conventional meat. As an initial exploration of nutritional engineering for cultured meat, Stout et al. have incorporated a biosynthetic pathway for carotenoids, a class of antioxidants native to some plants but not to animals, into primary bovine satellite cells (BSCs) [88]. They demonstrated that these carotenoid-producing BSCs showed antioxidant capacity and reduced lipid oxidation, potentially increasing the nutritional value of meat and reducing the link between red meat consumption and colorectal cancer [88]. There is a wealth of possible research in tuning the nutritional characteristics of cultured meat, even including edible therapeutics [88].
Source
