Effects of treatments on viability and culturability of L. monocytogenes
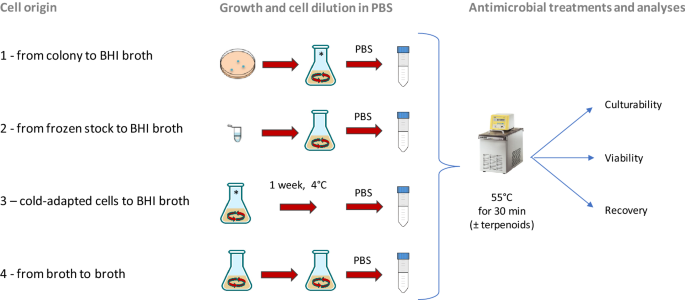
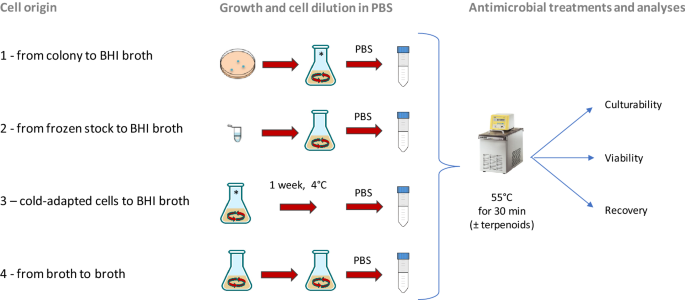
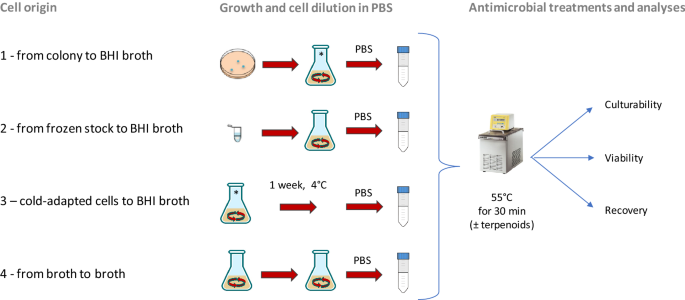
To examine the impact of cell origin on the efficacy of mild heat treatment combined or not with terpenoids, L. monocytogenes cells were cultivated in different conditions (Fig. 1). After growth, cells diluted in PBS were subjected to the antimicrobial treatment, in presence or not of terpenoids at sublethal concentrations, based on the previous determination of minimum inhibitory concentration (MIC)6. The results obtained are summarized in Table 1. In control cells no significative differences in terms of Active Fluorescent Unit (AFU)/ml and CFU/ml (viability and culturability, respectively) were observed, confirming an initial inoculum of approx. 6 log AFU or CFU/ml.
Schematic representation of four cell origin used in this study. Origin 1: from single colony to BHI broth; origin 2: from frozen stock to BHI broth; origin 3: cells derived from the condition 1 and then cold-adapted (1 week at 4 °C); origin 4: from culture in early stationary phase to BHI broth.
Table 1 Viability, culturability, and recovery in BHI broth of L. monocytogenes cells after mild heat treatment combined with terpens.
Based on flow cytometry data in non-treated cell suspensions we did not highlight relevant differences in terms of distribution of the 3 subpopulations (AFU, damaged and non-Active Fluorescent Unit non-AFU), indicating that the cell origin did not affect the viability of the cell, even after one week of storage at 4 °C. After the thermal treatment at 55 °C, the viability was not significantly affected in all tested condition. By contrast, the culturability was severely compromised. In fact, a decrease of culturability of 3.14, 2.66 and 2.57 log cfu/ml was observed for cell origin 2, 3 and 4, respectively (Table 1). Conversely, cells derived from condition 1 (from colony) were the less affected by the thermal treatment, with a decrease of culturability of about 1.90 log cfu/ml. The thermal treatment in presence of thymol and carvacrol increased the discrepancy between viability and culturability. Indeed, the viability decreased from 0.36 up to 1.34 log AFU/ml for cells from origin 2, 3 and 4. Conversely, cells from condition 1 was not significantly affected by the treatment. In parallel, the culturability was almost or completely abolished. Indeed, the culturability of cold-adapted cells (condition 3) was about 1.97 log cfu/ml and below the detection limit (< 1 log CFU/ml) in conditions 2 and 4. Conversely, the culturability of cells derived from colony to broth (condition 1) was less affected compared to the other conditions (3.08 log CFU/ml).
The synergistic effect between mild thermal treatment and antimicrobials has already been demonstrated for several microorganisms and conditions. Concerning L. monocytogenes, a relevant reduction of the treatment time required to obtain the same cell inactivation has already been reported in the presence of thymol, carvacrol, citral and (E)-2-hexenal6,11 and the synergy between essential oils and thermal treatment on foodborne pathogens has been recently reviewed by Gurtler et al.12. In first instance, the results obtained in these trials demonstrated that L. monocytogenes cells grown in aerobic conditions (stirred in a flask at 150 rpm) had reduced susceptibility than those grown in static conditions6, underlying the importance of the cell cultivation on the effectiveness of antimicrobial treatments. On the other hand, relevant differences in growth and survival performances of L. monocytogenes in relation to presence/absence of oxygen are well known21,22. In addition, as observed by Kragh et al.15, it is often assumed that a liquid batch culture contains phenotypically homogenous cells. However, these authors demonstrated that the inoculation method (i.e. cell history) has a relevant effect on the phenotypes of the resulting population. They demonstrated also that some characteristics (in the specific case the frequency of aggregation of Pseudomonas aeruginosa) of the cultures first seeded can be inherited in second generation cultures modifying responses to stress such as the presence of antibiotics.
In any case, the discrepancies between viability and culturability expressed as log CFU/ml raise the question of the viable but not culturable cells (VBNC) that in some cases consist in several log cells/ml. As stressed by Donnelly and Diez-Gonzalez23, improvement in testing methods for L. monocytogenes are needed to ensure an adequate sensitivity of detection for identifying and control the presence of this pathogen. Particularly, a better understanding of factors affecting the recovery and growth of VBNC cells is needed to determine the real effect of an antimicrobial treatment. Indeed, the VBNC state may be reversible (“resuscitation”) and this condition is a concern when it involves storage of food material23.
Studies regarding the efficacy of inactivation methods on L. monocytogenes reported similar results that raised the same issues. For example, Alessandria et al.24 observed that after a treatment with cold atmospheric pressure plasma (APP) most of the culturable cells were inactivated but, when inoculated in BHI medium (therefore removing the environmental stress), some of them were able to regrow.
Although it is still matter of discussion if this regrowth is the result of a true resuscitation phenomenon or it is rather due to few residual culturable cells25, these observations highlight the need to integrate culture-dependent and culture-independent approaches to monitor this pathogen, since conventional culture methods can overestimate the efficacy of the treatments performed.
The same considerations were reported by Noll et al.26, whose study evidenced that L. monocytogenes adapted to antimicrobial agents (in that case benzalkonium chloride) were viable and metabolically active according to FCM analyses but not detectable by standard cultivation techniques, indicating the occurrence of VBNC cells. Gu et al.27 investigated the susceptibility of some foodborne pathogens (including L. monocytogenes) to sanitizers such as free chlorine and peracetic acid. They hypothesized the induction of a VBNC state, already reported in literature for these chemicals28, because of the discrepancy between the inability to detect cells by plating and the results of PMA-qPCR and laser confocal microscopy, that evidenced large amounts of DNA from theoretically viable bacterial cells. However, the conditions adopted in that study did not allow resuscitation of this population in vitro, but a possible recovery in vivo cannot be excluded, raising alarming threats for food safety.
Effects of antimicrobial treatments and cell origin on recovery ability and growth kinetics
To test whether the cell origin could influence the ability of the cells to recover after antimicrobial treatment, stressed cells were inoculated in a 96-well plate (for a total of 28 wells/repetitions for each condition) for 48 h at 37 °C. The recovery was expressed as percentage of wells displaying growth on the total of inoculated wells (Table 1). As expected, 100% of the wells inoculated with non-treated cells of L. monocytogenes displayed growth, regardless of the cell origin. Concerning the ability to recover after the treatment at 55 °C, all cells were able to recover but to different extent. Only cells from condition 1 and 3 displayed growth in 100% of the inoculated wells. Conversely, less efficient was the recovery of cells from condition 2 and 4, with percentages assessed at 79 and 50%, respectively. When cells were subjected to the thermal treatment combined with terpenoids, the recovery capability was strongly impacted with an evident influence of the cell history. Cells from condition 2 were not able to recover after treatment. Conversely, the recovery of cells from conditions 3 and 4 was 43 and 11%, respectively. Instead, all wells inoculated with cells derived from condition 1 displayed cells growth. These differences can be explained by the culturability of treated cells (expressed as log cfu/ml). In fact, in the sample treated at 55 °C, the dilution used for the inoculation of wells (1:100) determined a mean inoculum of culturable cells of about 1 log cfu/ml or even less (e.g. the case of cells from condition 2 after treatment at 55 °C; all cases after the mild heat treatment combined with terpenoids). The variability associated with these low inoculums could explain the presence of wells with no visible growth. By contrast, considering the sample thermally treated in the presence of terpenoids, the growth percentage did not reflect the associated culturability. The growth observed could be due to unculturable cells which however resulted alive according to the FCM protocol. This consideration opens the question of the fate of these cells over time.
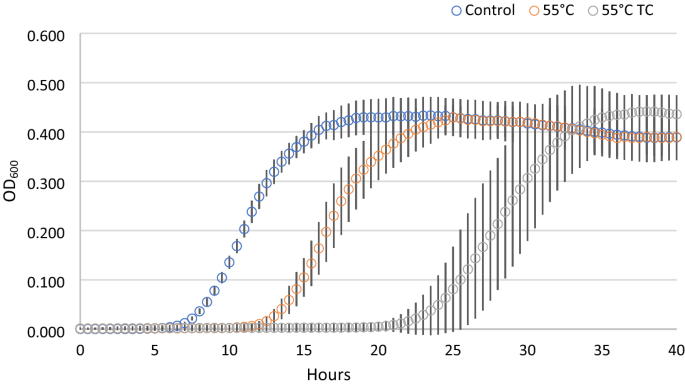
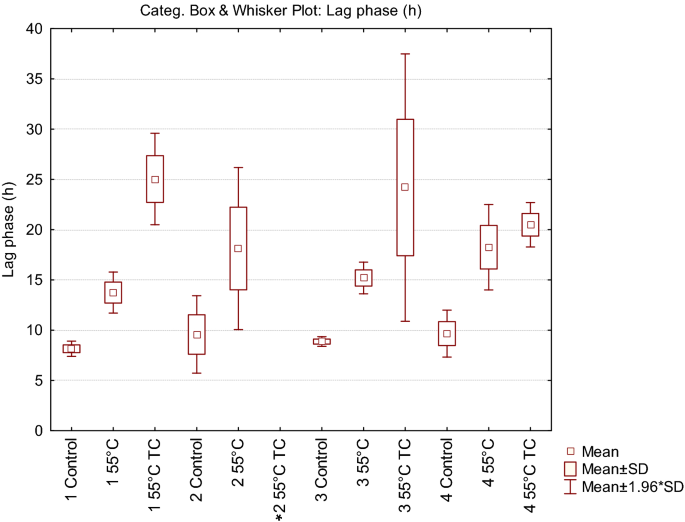
In parallel to the recovery ability, we investigated the kinetic parameters of the treated cells deriving from condition 1, compared with untreated control cells (Fig. 2). Each point of the growth curves represented the mean of the observation recorded in 28 experimental replicates. It is evident the delay in the growth curves induced by the thermal treatment. Even more noticeable was the effect on the lag phase lasting of the thermal-treated cells in presence of the two terpenoids. In addition, the analysis of the standard deviation of all the reading points of the growth curves showed how the variability was dependent on the kind of antimicrobial treatment (55 °C TC > 55 °C > Untreated). The data concerning the samples in which growth was observed were then modeled with the Gompertz equation, as modified by Zwietering et al.29. The measurement of the OD is an indirect method in which the absorbance changes occurs when the bacterial concentration reaches a minimum bacterial concentration of approx. 7 log cells/ml, after which the OD linearly increases up to a concentration of 8 log cells/ml reaching the higher absorbance allowed by the instrumental conditions30. The parameter λ of the model (lag phase duration) was used to estimate the time needed to obtain this critical threshold, i.e. necessary to have an increase of the OD600 baseline. Figure 3 reports the Box and Whisker plots regarding the λ values estimated in the wells in which growth occurred. Independently of the inoculum, λ increased, as expected, with the severity of the treatment. Noteworthy, also the variability of the responses increased with the stress applied. This variability was particularly relevant in the thermal treatment in the presence of terpenes, with the exception of inoculum 4 (inoculum from a liquid culture to fresh medium); however, in this latter case the data are affected by the presence of only 11% (3 out of 28) of positive cases. The lack of homoscedasticity of the variance did not allow the use of ANOVA to exploit the difference among the samples. Even after a log transformation of λ the requisite was not accomplished (data not shown). For this reason, the non-parametric Kruskal–Wallis test31 was applied and the results are reported in Table 2. The result of this test showed a significant difference between the ranks of λ and the treatments (P < 0.001). Untreated samples were not significantly different with the exception of inoculum 1 (direct inoculation from colony to broth) which showed lower λ values. Among the thermal treated samples, no differences were observed between inoculum 2 (direct inoculation from frozen stock) and 4 (inoculum from early stationary phase culture to fresh medium), which, however, showed respectively 79 and 50% of growth among the inoculated samples. Inoculum 1 and 3 (cold adapted cells), in which all the replicates grew, showed significantly lower λ values. The samples added with thymol and carvacrol were grouped together, even if the samples from inoculum 4 did not present significant differences with the samples obtained from inoculum 2 and inoculum 4 thermally treated at 55 °C. Nevertheless, in this latter case, only in 3 out of 28 samples the growth was observed.
Growth curves of cells derived from condition 1, after antimicrobial treatments. Each time point is the mean of 28 replicates. For each point standard deviation is reported.
Box and Whisker plot describing the variability of lag phase (λ) in relation to the different cell origin and treatment. In the condition marked by an asterisk no growth was observed in any of the 28 replicates.
Table 2 Results of the application of the non-parametric Kruskal Wallis test to the lag phase (λ) estimates in the different conditions.
Recovery of L. monocytogenes cells in food model systems
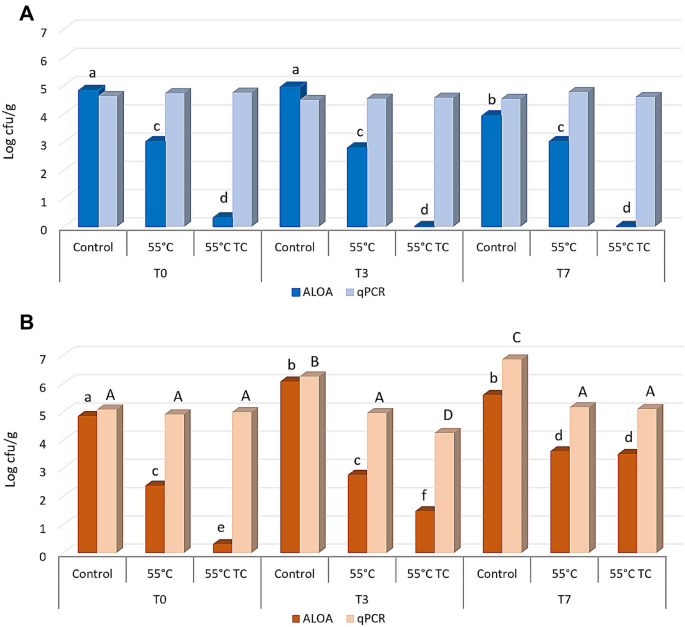
Based on viability, culturability and recovery data, the cells deriving from inoculum 1 (direct inoculation of a colony in liquid medium and growth for 24 h) resulted the more prompted to counteract the applied stress. To mimic the worst scenario, these cells were used to contaminate 2 different food matrices. The attention was focused on Gorgonzola cheese and sliced roast beef, which can be susceptible to L. monocytogenes colonization32,33. Both matrices were inoculated with non-treated (control) and treated cells (55 °C and 55 °C TC). The size of inoculum was around 4.6–4.8 log cell/g (50 µl of the original cell suspension in PBS) and after inoculation the samples were stored at 4 °C, then analysed after 3 and 7 days of incubation. Microbiological analyses were carried out using plate counting as well as qPCR to determine culturability and the total L. monocytogenes cell counting, respectively. A non-inoculated sample was also considered to exclude the presence of L. monocytogenes in the raw materials. The results indicated that this pathogen was not detected, neither by cultivation nor molecular methods, in non-inoculated samples for both kind of foods (data not shown). Results concerning roast beef slices (Fig. 4A) showed that at T0 the counting in the control sample was 4.81 log CFU/g (according to plate counting) and 4.61 log cells/g (according to q-PCR). In the treated samples, the qPCR confirmed the same cell concentration, but the culturability decreased at 3.0 log CFU/g in the thermal treated cells. The presence of terpenoids determined a higher decrease of the culturability (below 1 log CFU/g) since T0. However, treated cells were not able to recover their culturability after 3 and 7 days of incubation. Moreover, the total number of L. monocytogenes cells (qPCR data) remained stable over the time and in all samples, including control cells. Our results are in accordance with Skjerdal et al.34, where authors did not find any growth when L. monocytogenes was inoculated in a challenge test on roast beef slices, confirming that roast beef is not an ideal substrate for the growth of the cells when stored at refrigerated temperatures.
Culturability on ALOA and total L. monocytogenes cell counting by qPCR with species specific primer on roast beef slices (A) and Gorgonzola rind (B) stored at 4 °C after contamination. Before the inoculum cells were not treated (Control), treated at 55 °C (55 °C) or treated at 55 °C in the presence of thymol and carvacrol (55 °C TC). Significant differences between samples according to ANOVA are indicated by lowercase letters for cell culturability and capital letters for cell counting by qPCR.
A different scenario was observed when cells were inoculated on Gorgonzola rind (Fig. 4B). The initial quantifications at T0 were similar to those obtained for roast beef, both in terms of log CFU/g and total number of cells/g. Conversely to roast beef, control cells of L. monocytogenes were able to increase the total cell concentration after 3 days at 4 °C (T3) reaching values of about 6 log cell/g (with both quantification methods). After 7 days, control cells reached up to 7 log cell/g according to qPCR, while the culturability remained comparable to T3. Concerning heat treated cells, only a slight increase of the culturability was observed at T3 and T7, while the total number of cells detected by qPCR remained constant. Analogously, the total concentration of the heat treated cells in presence of terpens remained constant, while some of the cells reacquired their culturability after 3 (T3) and 7 (T7) days at 4 °C (Fig. 4B), reaching up to 3 log CFU/g. These results suggest that the increased values observed could be due to a resuscitation of VBNC cells rather than the cell ability to duplicate on Gorgonzola rind.
Differences among the two matrices can be explained by the competition between spiked cells of L. monocytogenes and the microorganisms naturally present on foods. This aspect was already underlined by Broady et al.33 which observed the inability of L. monocytogenes to grow, even if inoculated, in some roast beef samples. McDonnell et al.35 observed a lower growth of this pathogen in roast beef if compared with other meat product, and a 2 log cfu/g cell increase required 4 weeks at 4 °C. On the other side, blue veined cheeses such as Gorgonzola, are favorable substrates for L. monocytogenes32,36 and lactic acid bacteria, which dominate the microbiota, show limited anti-listerial activity, especially if the contamination takes places after the ripening period37.